 From the Access Research Network
From the Access Research Network
At www.arn.org/docs/odesign/od201/peeringdbb201.htm
Origins & Design
Archives
Feature Article
Origins & Design 20:1
Issue 38
Peering into Darwin's Black Box:
The cell division processes required for bacterial life
Joseph W. Francis
Associate Professor of Biology
Cedarville College, Ohio
francisj@cedarville.edu
Abstract
The smallest living
building block of life, the cell, is enormously complex, and a great number of
its mechanisms are irreducibly complex. Few theories have been proposed
explaining how irreducibly complex mechanisms could have evolved by Darwinian
natural selection. It could be argued that given enough time a simple
reproducing population of living “protocells” could have provided a format
for the evolution of complex mechanisms. However, even in “simple” bacteria,
the most basic cell functions display irreducibly complex mechanisms—for
instance, cell division. This article considers the origin of an irreducibly
complex cell division apparatus and contrasts protocell theory with intelligent
design theory.
“The only life we know for
certain is cellular.....”
— Harold
J. Morowitz
Protocell theory is a
popular theory often proposed to explain how biochemical complexity arose in
living cells by completely natural, evolutionary processes. The theory
postulates that the complex cells we observe today evolved gradually from
simpler protocells via natural selection. For example, Harold Morowitz has
suggested that the original protocells were unstable and prone to
self-destruction; but through the continual formation of billions of protocells
over millions of years, eventually a stable, more advanced, protocell formed (Morowitz,
1992).
In contrast to
protocell theory, intelligent design theory postulates that some biochemical
mechanisms within cells are irreducibly complex, which implies that they are not
products of any gradual, naturalistic process of formation. For example, many
cell mechanisms resemble preassembled machines containing interdependent parts
that work together to perform a cell function. Since all the co-dependent parts
must be present before the mechanism is capable of function at all, it is
unlikely that the mechanism evolved by any gradual process, but instead appears
to have been designed.
Examples of irreducibly
complex biological systems have been documented by biochemist Michael Behe in Darwin’s
Black Box (Behe, 1996). Behe defines irreducible complexity as “a single
system of several well matched, interacting parts that contribute to the basic
function, where the removal of any one of the parts causes the system to
effectively cease functioning.” As one of several examples, Behe cites the
bacterial flagellum, a whip-like cell appendage that has a complex motor
apparatus at its base. How did such a complex structure evolve in what is often
described as a “simple” cell? Protocell theorists propose that the bacterial
flagellum evolved gradually by natural selection (Loomis, 1984), implying that
such complex mechanisms are late developments in the evolution of bacteria and
would not have been present in early protocells.
If, however, the
protocell is a self-reproducing cell, as the theory suggests, then several
essential cell functions that appear to be complex in contemporary cells would
have to be present even in the early protocell. For instance, a cell division
mechanism would be essential even in early stages of protocell evolution. The
impression given in many biology textbooks is that cell division is a simple
process. However, upon close examination it becomes clear that cell division
even in bacteria is a complex cellular process. This raises several questions
concerning protocell theory. For instance, how did a simpler cell division
mechanism function? How did it lead to the complex mechanism we observe today?
Are any remnants of a simpler cell division process evident in cells today? Is
the cell division process irreducibly complex?
The existence of an
irreducibly complex cell division process would present two problems for
protocell theory. First, it would require the theory to explain the origin of a
protocell that must possess, at a minimum, an irreducibly complex cell division
process for survival. Second, if a protocell is capable of surviving with a
simple cell division process, how does natural selection lead to a more complex,
indeed an irreducibly complex, cell division mechanism?
In considering these
questions, I will examine cell division in bacteria. There is general agreement
among biologists that bacteria represent a late protocell, or at least an
evolutionary link between a simpler protocell and eukaryotes, based on the fact
that bacteria appear to be simple cells, both morphologically and genetically,
compared with eukaryotes. Bacteria are also well adapted to independent
unicellular life and hostile environments, conditions postulated to exist on the
early earth. However, the assumption that bacteria are simple is itself open to
question, as we shall see. I will focus on one primary question: Is there a
minimum set of reactions a bacterium must possess to divide and reproduce, and,
if so, are these mechanisms irreducibly complex?
The basics of cell division
Let’s begin by
listing the essential processes a bacterium must possess to reproduce
effectively. For the sake of simplicity, we will ignore for the time being the
requirement for energy in the form of ATP and its biochemical production in the
cell.
- DNA replication.
Without duplication of the molecule that contains the genetic code, life
cannot continue.
- Cytokinesis. This
involves division of the cell housing, which includes the cytoplasm and
membrane components. The cell housing must be manufactured and assembled before
the cell divides, otherwise the newly replicated cells would continually
reduce in size with each division.
- Protein synthesis.
The cell must make or acquire proteins since DNA replication and membrane
production both require enzymatic processes.
- DNA segregation. The
cell must have a way to partition its DNA equally among the new offspring.
- Coordination between
DNA replication and cytokinesis. The two basic steps—DNA replication and
cytokineses—must be coordinated, otherwise the cell cytoplasm and membrane
could divide before the DNA replicates, leading to anucleated, nonviable
offspring or cells with multiple chromosomes (polyploidy).
The common link among
all the processes mentioned above is the need for proteins. In fact, if protein
synthesis is inhibited, cell division ceases (Lewin, 1997). Therefore, unless
there is a source of pre-manufactured proteins that can be transported into the
cell, the cell itself must contain a protein synthesis process. Even in
bacteria, protein synthesis is a highly complex and regulated process, involving
many proteins and machine-like protein complexes. More than 200 hundred proteins
involved in this process have been identified in the bacterium Escherichia
coli (E.coli) (Javor, 1998). Without this protein synthesis
machinery, bacteria would not be able to divide (nor even keep the cell’s
“house” in order).1
Is cell division irreducibly
complex?
Let’s now consider
our central question: Are the complex biochemical processes that control cell
division representative of a minimal set of reactions that the cell requires for
life, and are they irreducibly complex? There is good evidence to suggest that
the process of cell division is indeed irreducibly complex, for the steps
involved are interdependent and highly coordinated. For example, crucial steps
such as DNA transcription require proteins (see Figure 1)—while protein
synthesis in turn is dependent upon transcription. Moreover, evidence suggests
that the processes involved in cell division are highly regulated and
coordinated in a sequential fashion. For instance, in bacteria, cytokinesis does
not proceed until DNA replication is complete, so that the DNA is precisely
partitioned into the developing daughter cells. Each process itself is complex
and if any one of the processes is inhibited, cell division ceases. This
interdependence fits the criteria of an irreducibly complex system.
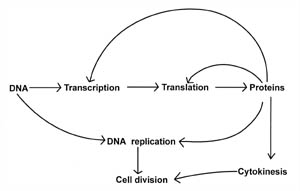
Figure 1. Relationship
between some of the basic biochemical processes that are required for life and
cell division. Transcription and translation are processes that are involved in
making proteins by deciphering the genetic code (DNA). However, proteins are
required for both the transcription and translation processes, as well as for
DNA replication and cytokinesis. Note that proteins play a vital role in each
cell process and serve as the major interconnecting link between each process.
Cell division is a protein-dependent mechanism.
Does this arrangement
also represent a minimal system that must be present in all cells, including any
hypothetical protocell? Or could it have evolved gradually? Let’s consider the
possible gradual derivation of the cell division apparatus in the protocell.
Cell division and the protocell
One of the more popular
theories of protocell evolution, presented in biology textbooks, involves the
encapsulation of the basic processes of biopolymer synthesis in a membrane
(Cooper, 1997). It is then postulated that the protocell began to divide by a
simple mechanism. In other words, it is assumed that all the cell functions
required for life, perhaps even those required for cell division, were
pre-manufactured and pre-functioning processes sequestered together by a cell
membrane. (One barrier to cell division that the early protocell would encounter
is that in an aqueous environment there is a natural physical resistance to the
membrane disruption needed for cell division. For the sake of discussion, we
will assume that the dividing protocell was in a membrane-disrupting environment
that promoted some type of membrane blebing or stressing so that new cells could
bud or pinch off the protocell.)
There are several
fundamental problems with the encapsulation theory. First, how does a
cytokinesis process develop before the membrane forms the cell? Cytokinesis
requires a membrane-enclosed cytoplasmic space and could only develop after
encapsulation. Yet in that case—if cytokinesis evolved only after
encapsulation—then it would have to evolve rapidly, otherwise the cell would
not reproduce and its long-term survival would be questionable. One possible
postulate is that the early cytokinesis process was a much simpler process
compared with the complex cytokinesis mechanism observed in bacteria today. That
would imply, however, that there was very little regulation or no coordination
between DNA replication and cytokinesis and other cell systems, which in turn
implies that the division of the membrane and successful transfer of genetic
material was haphazard and inefficient. The protocell would partition its DNA
into new daughter bacteria, and then divide, by random uncoordinated processes.
Let’s examine some
hypothetical protocell models that involve cell division without coordination
between the processes of DNA replication, protein synthesis, and cytokinesis. To
keep the model simple we will portray the early protocell as monoploid—i.e.,
containing one strand of DNA (though some researchers suggest that the early
protocell may have been polyploid, harboring short pieces of DNA).
In contemporary
bacteria, DNA replication precedes cytokinesis so that a single cell momentarily
has what appear to be two copies of circular DNA; thereafter the DNAs are
partitioned into two new daughter bacteria, resulting in two separate daughter
bacteria with one circular DNA molecule each. If, however, in the early
protocell there was no coordination between cytokinesis and DNA replication, we
can predict two scenarios for how early protocell division could have occurred:
either DNA replication occurred at a faster rate than cytokinesis, or,
conversely, cytokinesis occurred at a faster rate than DNA replication. If
cytokinesis occurred at a faster rate, the result would be the production of
many anucleate, nonviable bacteria. The parent cell would accumulate DNA and
become polyploid with the potential occasionally to produce daughter bacteria
containing DNA (see Figure 2). Alternately, if DNA replication preceded
cytokinesis, then greater numbers of viable offspring would be produced at a
faster rate. However, in this case once again DNA would accumulate in some
bacteria due to unequal partitioning, leading to polyploidy. It is interesting
to note that a population of both monoploid and polyploid bacteria seems to be a
common outcome for all the predicted protocell division scenarios.
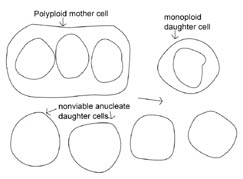
Figure 2. Hypothetical
model of protocell division. In the protocell, DNA replication and cytokinesis
would not be coordinated. Therefore DNA replication could occur faster or slower
than cytokinesis. This figure shows a potential outcome of cytokinesis occurring
at a faster rate than DNA replication. Note that many non-viable, anucleate
daughter bacteria would be produced, as well as bacteria that are monoploid or
polyploid. An animated version of this figure is available for viewing at: http://www.cedarville.edu/dept/sm/jwf/division.htm.
Polyploidy: The problem of a
full house
Since polyploidy is
predicted to be a common outcome of protocellular life, and it since is
generally detrimental to cellular life in contemporary cells, it is important to
consider its effects on protocell evolution. Polyploidy would present at least
two major hurdles for the evolving protocell. First, it would mean a diminished
capacity for natural selection of favorable traits. For instance, Koch has
calculated an upper limit for the number DNA copies the protocell could
reasonably contain and still flourish. His upper limit for DNA is based on the
fact that favorable mutations would be diluted in the “selfish” protocell
carrying great numbers of chromosomes and duplicate genes (Koch, 1984).
Another problem that
polyploid protocells would face is regulation of cell volume. The cell volume
would eventually have to adjust to accommodate the increase in DNA and a
corresponding increase in protein production. (In contemporary organisms that
successfully harbor polyploid cells, those cells are larger than its typical
cell.) How does the protocell adjust its size if there is no coordination
between membrane events and biopolymer production? Perhaps the cell could
inhibit uncontrolled DNA replication and protein accumulation by the production
of an inhibitor of gene expression. But in that case, what would control the
inhibitor so that it would not inhibit gene expression of all DNA, especially if
the inhibitor is concentration-dependent and is transferred to a daughter cell
with fewer DNA strands? We could postulate that an inhibitor would require a
complex mechanism to ensure that gene expression from at least one DNA is not
inhibited.
Despite these problems,
is it possible that a viable bacterium with either a single DNA chromosome or
several DNA chromosomes could be consistently produced throughout these
reproduction cycles? Certain mathematical models show that this is possible,
though production of nonviable cells would be common. It is also possible that
this stochastic cell division process would create the condition we find in
nature—namely, the continual production of bacteria that contain only one DNA
molecule (monoploidy). However, the question remains how the unregulated
division process of the protocell would lead to the highly organized and
controlled division process that we observe in bacteria today.
The clockwork of cell division
To answer that
question, we need to focus for a moment on those highly organized and regulated
processes observed in the majority of bacteria today. Biochemical analysis
reveals a bacterial cell division process that operates with remarkable
precision. For example, E.coli bacteria replicate their DNA every 40
minutes (Lewin, 1997). In wild type E.coli, DNA replication and
cytokinesis each occur at fixed time intervals (the latter takes 18 to 20
minutes), and the entire cycle is repeated with clockwork-like precision each
time a bacterium divides. However, E.coli exposed to favorable conditions
(when resources are plentiful) can divide as fast as 18 minutes, because the
cell can overlap the fixed processes. That is, DNA replication can begin twice
before the cell membrane divides, such that the new daughter bacteria receive
DNA that is in the process of being replicated.
What is the signal for
this increased rate of response? The trigger mechanism is unknown, but what is
known is that bacterial cell division is coordinated precisely with the increase
in bacterial cell mass. If the rate of cell growth is fast, the cell division
mechanism responds by cycling at a faster rate. As a recent review article
notes, “It is surprising that genetics, which has been a powerful tool in
unraveling other regulatory circuits, has not yet been exploited to elucidate
how E.coli regulates its mass” (Vinella, 1995). One theory suggests
that as the cell increases its mass, it also increases the concentration of an
“initiator” protein that triggers the cell cycle. Even though it is not yet
clear how such an initiator may work, many of the events surrounding the
initiation of both DNA replication and cytokinesis are being elucidated.
To better understand
how both of these processes are coordinated and regulated during cell division,
let’s take a look at what is known about the initiation of both of these
events.
Initiation of DNA replication
The goal of DNA
replication in bacteria is the duplication of a circular DNA strand. Once DNA
replication begins, the cell is committed to complete the process. The primary
player in DNA replication is the DNA polymerase protein, which is a fairly large
and complex protein that works in coordination with DNA to unwind proteins.
Coordinating with it is a specialized ring-clamp protein that can literally
glide up and down the DNA and help keep the DNA polymerase tethered to the
DNA—an apparatus referred to as the replisome. How does the DNA polymerase
“know” when and where to assemble the replisome?
Before the polymerase
can find its start site, two problems must be solved: the DNA must unwind, and
single-stranded DNA must be exposed. The unwinding and stabilization of single
DNA strands involves an elaborate arrangement of proteins called an initiation
complex, which assembles at a unique site on the DNA (see Figure 3). A protein
designated DnaA is the most crucial protein involved in the initiation complex
and it is found in many bacteria. DnaA recognizes specific nucleotide sequences
and binds to them near a site on the DNA called the origin of replication.
Individual DnaA proteins also have the special property of being able to bind to
one another, and they exploit this ability by forming a cluster of up to 40
monomers, which causes the DNA to bend around the cluster. The bending stresses
the structure of the double helix and several regions that are rich in A-T base
pairs open up, exposing single strands of DNA. (A-T base pairs are weaker than
G-C base pairs). However, the unwinding of the DNA causes tension in the DNA
double helix, because the entire bacterial DNA chromosome is circular. This
tension is relieved by two other proteins present in the initiator complex: DnaB
and DnaC. This complex of DnaB and DnaC is so large that it appears as a blob
when visualized with the aid of an electron microscope. It is often referred to
as the engine of initiation.
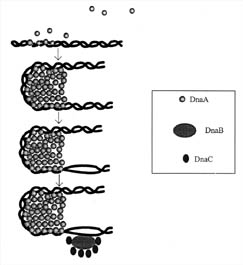
Figure 3. Initiation
of DNA-replication in prokaryotes. DnaA protein binds to repeated sequences on
the DNA near a site called the origin of replication. These repeated sequences
are conserved and are called consensus sequences. The consensus sequences that
the DnaA protein recognizes are of two classes, those containing nine
nucleotides and those containing thirteen. The self-association of the DnaA
monomers forms a cluster that causes the DNA to distort and bend. The bending
causes unwinding of the DNA. The unwinding continues with the aid of three other
proteins, Helicase (DnaB), DnaC, and gyrase. Single-stranded DNA is also
stabilized by a protein called SSB (not shown). (Based on Figure 15.18 in Lewin,
1997.)
The initiator engine
eventually displaces the DnaA and promotes the continued unwinding of DNA
through the specific action of DnaB, which is a helicase. Helicases are
ATP-dependent enzymes that break hydrogen bonds and can unwind DNA at a rate of
500-1000 base pairs per second. Helicases are classified as motor proteins,
which are enzymes that convert chemical energy into physical movement. E.coli
has 12 distinct helicases. The DnaB helicase works in conjunction with another
protein called gyrase, which is part of another family of proteins essential for
cellular life called topoisomerases. Gyrase has the remarkable ability to cut,
unwind, and then rejoin DNA strands, relieving the tension created by the
helicase-induced unwinding.
The DnaB helicase
creates an interesting problem. The unwound single-stranded DNA is much less
stable than the double-stranded form and it can potentially bind to itself.
Another protein called single-stranded binding protein (SSB) binds to the
strands and stabilizes them. The SSB proteins are required for replication, and
a mutation in the gene for their production is lethal.
It certainly appears
that several crucial factors must be in place for successful initiation of DNA
replication, and moreover it appears that they work together in an irreducibly
complex fashion.
A possible objection to
the conclusion of irreducible complexity is that DNA replication can be
performed in a cell-free system by adding back just a few components of the
replication machinery. This procedure known as PCR (polymerase chain reaction)
is a simple and powerful way to increase the concentration of isolated DNA in
the laboratory. The procedure requires the use of DNA polymerase but does not
require DnaA, helicase, or gyrase. One might conclude, therefore, that these
components are not needed and that DNA replication can be achieved by a simpler,
possibly non-irreducibly complex mechanism.
However this is not the
case; the PCR reaction involves procedural steps that essentially replace the
functions of the missing enzymes. For example, high temperature is used in the
PCR reaction to unwind and “unzip” the DNA—essentially replacing the
functions performed by the helicase and gyrase enzymes. Thus the same functions
are always required for DNA replication, even if they are achieved in different
ways.
The initiation of cytokinesis
Cytokinesis involves
the coordination of many interacting components and must perform several major
feats, including the synthesis of the membrane and cytoplasmic components
required to create two bacteria from one. The synthesis of membrane and cell
wall is quite an accomplishment considering the fact that many bacteria possess
three outer layers: cytoplasmic membrane, cell wall, and outer membrane. During
cell division, all three of these layers must be precisely extended in a short
period of time, since production of daughter bacteria that are the same size and
shape as the parent cell can occur rapidly.
Moreover, a majority of
these new membrane and cell wall components are manufactured preferentially near
the dividing point of the parent cell and are coordinated with constriction of
the cell at the same location. The cytokinesis process also accurately
partitions the DNA into each daughter cell, before the division of bacteria is
completed. The DNA segregation mechanism is incredibly accurate, resulting in
correct partitioning of the DNA greater than 99.9% of the time (Vinella, 1995).
The initiation of
cytokinesis centers around a region on the membrane that will eventually become
the dividing plane of the cell or the septum. Studies involving E.coli suggest
that the septum is derived from a site on the membrane called the periseptal
annulus. The periseptal annulus is a ring that encircles the cell and appears to
be the result of an invagination or a melding of the inner membrane and the cell
wall. The septum forms near this ring exactly at mid-cell. (It is unknown how
the cell precisely measures the exact center of the cell.) Several proteins
involved in septum formation form a complex at this site, working together to
form a constriction ring, synthesize new membrane and cell wall, and break old
membrane and wall attachments.
One of the earliest
acting and most crucial proteins involved at this site is the FtsZ protein. The
FtsZ protein self-polymerizes and is the primary component of a division ring
that is hypothesized to constrict during cytokinesis. Upon completion of the
septum, FtsZ depolymerizes. There is strong evidence to suggest that FtsZ is an
essential cell division factor for free-living bacteria (Vincente and Errington,
1996). (Bacteria are considered to be free-living if they are capable of
independent life free from a host organism or do not require complex nutritional
factors that are typically supplied by the host organism.) The role of FtsZ in
cell division is supported by the fact that it has been found in bacteria as
diverse as mycobacteria and archaebacteria (Baumann and Jackson, 1996). Some
studies report that it has some structural and functional similarities to the
cytoskeletal proteins found in eukaryotes (Vincente and Errington, 1996). It
also has the ability to self-polymerize into strands and cyclic structures in
vitro (Erickson, 1996). The FtsZ protein concentration is regulated at the level
of transcription and its concentration is estimated to be between 5000 and
20,000 molecules per cell. Some studies have suggested that the concentration of
FtsZ fluctuates with the cell cycle and its concentration can change by as much
as 50 percent.
Recent studies are
uncovering a fascinating story about the biology of the FtsZ protein. The data
show that FtsZ is regulated both temporally and spatially by transcription and
inhibitor proteins. Most of the data for this system has been derived from
studies of E.coli. The precise regulation of FtsZ is supported by several
studies showing that a critical amount of FtsZ is needed for cell division, and
that its overproduction or underproduction can cause cell division anomalies and
affect viability (Lutkenhaus, 1993).
Let’s examine some of
the proteins with which FtsZ interacts, several of which are located on the cell
membrane at the mid-cell septum. Two proteins located at the E.coli
septum, ZipA and FtsA, are required for proper FtsZ function and may be directly
involved in regulating its actions (Ma, 1996). For instance, a regulatory role
for FtsA is supported by the fact that a certain ratio of FtsZ/FtsA is required
for cytokinesis to proceed. Any deviation from the critical FtsZ/FtsA ratio
causes inhibition or alteration of cytokinesis.
There are several
hypothetical models for how FtsZ could act to constrict the cell at the septum
(Figure 4). One of the more intriguing aspects of FtsZ biology involves how it
preferentially forms the constriction ring at the mid-cell septum. FtsZ has
several other septal site choices for binding because multiple binding sites are
present in a single bacterium. For instance, in a pre-division mother cell, new
periseptal annuli are predicted to form very early on either side of the
original annuli and are eventually placed at one quarter the length of the cell
on each side of the mid-cell septum. These potential FtsZ binding sites will
form the mid-cell septa in the newly formed daughter bacteria (see Figure 5). In
addition, there are septum binding sites at the poles of the bacteria, since the
poles were derived from septa from earlier cell divisions (see Figure 6).
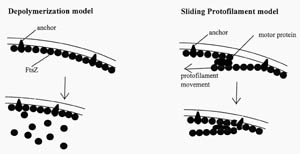
Figure 4. Two
hypothetical models for how FtsZ could act at the septum. FtsZ is known to
self-polymerize in vitro, forming rings and filaments. In the depolymerization
model, FtsZ constricts the cell by polymerization-depolymerization cycles. In
the sliding protofilament model, FtsZ protofilaments slide past one another.
(Redrawn from Figure 8, Bramhill, 1997.)

Figure 5. Derivation
of the septum and periseptal annuli. The periseptal annulus, which is formed
when the cytoplasmic membrane and the cell wall meld together, forms a ring
around the cell precisely in the middle of the cell. It is hypothesized that the
annulus serves as a site for development of the septum. New periseptal annuli
have been detected forming very early in the life cycle of the bacterium and
move away from the center annulus as the cell grows. They move to a position
mid-way between the cell pole and the mid-cell annulus. They are then in
position to serve as the mid-cell annulus of the newborn daughter bacteria.
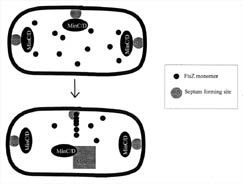
Figure 6. FtsZ is
directed to the mid-cell septum by the combined actions of several proteins.
Each bacterium has several septum locations where FtsZ can bind. Both poles of
the bacteria contain septum complexes, since they were derived from mid-cell
septa from their mother bacteria. FtsZ is directed toward the mid-cell septum
region by the combined action of the MinC, D, and E proteins. The MinCD protein
complex inhibits FtsZ binding at the poles and MinE overrides the inhibition at
the mid-cell septum, allowing FtsZ to bind and polymerize. It is not known how
MinE chooses the mid-cell septum.
How does the FtsZ
protein discriminate between these sites? It is now believed that a set of
proteins generated from a single genetic locus, the MinB locus, are involved in
directing the FtsZ protein to the mid-cell septum. Three proteins from MinC,
MinD, and MinE genes work together to inhibit cell division at the cell poles
and promote it at the mid-cell septum. The data and current models show that the
MinC and D proteins act together as an inhibitor and prevent the FtsZ protein
from acting at any septal site. The MinE protein counteracts the effects of the
MinC and D proteins precisely at the mid-cell, allowing the FtsZ protein to bind
and polymerize there (see Figure 6). Apparently it is the ratio of MinE to MinCD
that is important, since any deviation from an optimal MinCD/MinE ratio causes
aberrant cell division. So, remarkably, FtsZ is controlled by the spatial
concentration of Min proteins (Lewin, 1997).
Let’s consider the
possibility that FtsZ and the Min proteins could have formed through natural
selection. How does natural selection, using the random, uncontrolled division
processes of the protocell, promote a cell division system that requires precise
amounts of several essential factors in the right location at the right time?
What is the selection pressure? Consider the FtsA, FtsZ, and Min proteins: each
protein is a required component of the cytokinesis process; if one factor is
missing, the cell does not divide properly. In fact, if the concentration of the
factors is altered, cell division and cell viability are affected. Therefore, if
evolution of all of the factors does not occur simultaneously, each factor alone
could be a liability to the cell rather than an asset. If each of the factors
alone is deleterious to life, then the evolution of each individual factor is
less probable, since the cell lineage harboring the factor would tend to die
out.
For instance, consider
the scenario whereby evolution of the MinC, D, and E proteins occurred before
evolution of FtsZ. Since it appears that one of the primary functions of the
MinCDE system is to control FtsZ, what do the MinCDE proteins do after they
evolve? We can postulate that the MinCDE proteins would be quite useless or even
deleterious in the cell without the presence of FtsZ unless they were originally
selected to perform another function.
Alternately, what if
FtsZ evolved first? This seems more likely since FtsZ seems to be essential in
all free-living bacteria. However, our current understanding is that FtsZ
requires several binding proteins and the MinCDE system to successfully promote
cell division at the mid-cell septum. Without the MinCDE proteins present in the
cell, FtsZ will polymerize at the poles of the cell and cause the formation of
anucleate mini-bacteria, diminishing the propagation of the cell lineage. This
is supported by studies which have shown that if the FtsZ concentration is
elevated in bacteria, it can overcome the MinCD mediated suppression of
septation at the poles of the cell, and aberrant division can begin at several
septal sites in the cell.
If FtsZ by itself has a
negative or lethal effect on the propagation of cell lineages, could it have
evolved in a dormant state before the evolution of its required co-factors? If
so, what is the selection pressure that promotes the evolution of a dormant or
inhibited FtsZ factor? The scientific evidence points to the fact that a MinCDE
or equivalent system is required for FtsZ to function properly and supports the
hypothesis that many factors would have to evolve rapidly and simultaneously for
FtsZ-dependent cytokinesis to proceed. This seems to violate the basic tenants
of Darwinian gradualism. Even if the MinCDE and FtsZ factors could have
co-evolved we are still left with questions involving how the MinCDE system can
select the mid-cell septum and how regulation of FtsZ polymerization occurs.
The fact that FtsZ
requires several protein factors that work in a precise interdependent fashion
to promote cytokinesis shows that the FtsZ-dependent cytokinesis mechanism
present in E.coli is an irreducibly complex system. As such, it is highly
questionable whether this complicated system could have arisen by Darwinian
gradualism starting with a simple protocell.
Evidence for a Darwinian
process in the late protocell
Since it appears that
it is unlikely that the MinCDE-FtsA-FtsZ-dependent cytokinesis apparatus found
in E.coli could have existed in the early protocell, a biologist
committed to philosophical naturalism could postulate that such a system may
have evolved in the prebiotic soup, or else in the more stable late protocell.
First, let’s consider
the derivation of the system by a gradual mechanism in the pre-biotic soup. This
seems even more unlikely than its derivation in the early protocell. For
instance, how could spatial control be achieved in the vast oceans of the
prebiotic soup? Dilution would certainly be a problem. In addition, there is no
membrane to divide, which is the primary reason for selecting such a system.
Once again we can conclude that the spatial and temporal control of several
factors involved in cytokinesis represents, at least in E.coli, a complex
system that is difficult to account for by any gradualist theory.
By contrast, there is
data that may support the evolution of the FtsZ-dependent cytokinesis system in
the late protocell. For instance, even though the FtsZ protein is highly
conserved, several bacteria lack some of the proteins that are part of the FtsZ-dependent
cytokinesis system (see Table 1). Could these bacteria represent cells that have
evolved only part of the FtsZ-dependent cytokinesis system or a simpler form of
the system? Could these bacteria thus be close descendants of the late protocell?
Table 1. Cytokinesis
proteins present in the genomes of free-living bacteria as detected by amino
acid sequence.
There is a one major
problem with this suggestion. Proteins can be identified either by their
function or by their amino acid sequence. Most of the missing cytokinesis
proteins in these bacteria have been determined to be missing because their
amino acid sequence is not found. But it is possible that a different protein is
fulfilling the same role as the missing protein. For instance, the amino acid
sequence of the MinE protein is not found in the bacterium Bacillus subtilis,
but a protein designated DivIVA has been detected that fulfills the role of the
MinE protein (Boche and Pichoff, 1998). Therefore, in B.subtilis the
cytokinesis apparatus appears to be irreducibly complex even though it lacks
MinE.
Using the Intelligent
Design model, we could predict that since an irreducibly complex FtsZ-dependent
cytokinesis mechanism exists in E.coli and B.subtilis, and since
it appears to be essential to bacterial life, a similar system may exist in all
bacteria. The components of the system could be different structurally but their
functions would be the same or similar. At this point, however, since all the
components have not been identified in all bacteria, all we can conclude is that
the cytokinesis apparatus of E.coli fits the definition of an irreducibly
complex system.
Since the complete
genomes of several bacteria are known, it would be interesting to analyze these
genomes by sequence analysis for the presence of components of the FtsZ-dependent
cytokinesis apparatus. This would allow us to begin to determine which
components exist and which have yet to be identified. This analysis would also
help determine the minimal requirements needed for a cytokinesis apparatus and
would represent a first step toward elucidating whether a simple or even
non-irreducibly complex cytokinesis system exists.
Evidence from amino acid
sequences
FtsZ has been detected
in all free-living bacteria analyzed for its presence. Based on data derived
from the study of E.coli we will hypothesize that the proteins FtsZ, FtsA, MinC,
MinD, and MinE/DivIVA represent a core cytokinesis apparatus. Table 1 shows
which of these proteins have been detected by amino acid sequence analysis in
the thirteen free-living bacteria whose complete genomes have been determined.
Five of the thirteen bacteria species possess a full complement of these
proteins. In the others, one or more of the proteins are missing. It is
interesting to note that at least one Min protein is present with FtsZ in all
the bacterial species. Could some of the Min proteins perform multiple roles, or
is one Min protein sufficient? We could argue that perhaps the combination of
FtsZ, one binding protein, and one septum-locator protein like MinE, represents
a minimal irreducibly complex cytokinesis apparatus in bacteria. At least, so
far the evidence points toward this conclusion. However some bacteria like
mycoplasma, which often require a host cell and complex growth requirements,
appear to be missing the amino acid sequence for a majority of these factors.
Therefore, until all bacteria are analyzed, and cytokinesis proteins detected,
we cannot make the claim that an irreducibly complex cytokinesis system is a
universal phenomena in all free-living bacteria.
Even though a universal
system has not yet been detected, genome analysis has revealed that the FtsZ
protein itself is an important universal cytokinesis protein because it is found
in all free-living and many non-free-living bacteria. This is intriguing because
non-free-living bacteria, such as the mycoplasmas, often borrow proteins or
energy from the host cell to survive and therefore they tend to have smaller
genomes and fewer proteins, and yet mycoplasma possesses the FtsZ protein.
However, Chlamydia trachomatis, a parasitic bacterium which requires
another cell in order to grow and divide, is the first bacterium in which the
FtsZ amino acid sequence has not been detected (Stephens et al., 1998). Even
more interesting, the FtsA and MinD genes have been located in chlamydia. Could
chlamydia harbor a cell division system that is FtsZ-independent, or a
simpler cell division system? Could it even represent a transitional bacterium
that has evolved only part of the cytokinesis apparatus?
Possibly. However,
there is data to suggest that chlamydia use a complicated cell division
mechanism and probably divide using a division ring like FtsZ. In fact, there
are three lines of evidence supporting this. One line of evidence shows that
chlamydia possess a cell division protein called cytoplasmic axial filament
protein (cafa). This protein has been shown to be essential to division in some
bacteria and may form filaments similar to FtsZ (Okada et al., 1994). Therefore,
cafa could replace FtsZ. Second, non-free-living bacteria like chlamydia have
been found to recruit cytoskeletal components from the cell they parasitize. One
researcher suggests that the recruitment of cytoskeletal components could allow
the bacteria to use the host cell proteins for stress fibers and cleavage rings
(Rhee and Sanger, 1994; and Sanger, 1999). It is possible that chlamydia could
operate in this manner. Third, cell division of chlamydia is affected (the cells
increase in size) when the cells are exposed to cytoskeletal disrupting agents,
suggesting that a cytoskeletal component is involved in division (Schramm and
Wyrick, 1995). At this point, we will have to wait and see if other cytokinesis
components will be discovered in chlamydia.
It also appears from
sequence analysis that, in general, parasitic bacteria with small genomes, like
mycoplasma, possess fewer of the core cytokinesis protein factors. However, it
is also interesting to note that in the bacteria Aquifex aeolicus and Thermotoga
maritima, two of the free-living bacteria considered by evolutionists to be
the most ancient, an almost-full complement of the core cytokinesis factors are
found (see Table 1). In fact, all the factors are found in Aquifex which has a
genome one-third the size of E.coli.
Speculative model for FtsZ
FtsZ is speculated to
play a wider role than just formation of the septum. For instance, it may play
an important role in the timing of cytokinesis. If FtsZ has other functions and
regulates other aspects of cell division, this would support the theory that it
(or cytoskeletal proteins like it) may be essential to cell division.
To understand how FtsZ
could play diverse roles in cell division we could speculate how it might
regulate the timing of cell division with an increase in cell mass. We could
hypothesize that daughter bacteria receive enough MinCD protein from the parent
to maintain inhibition of division at all the septal sites of the cell. As the
cell mass increases, so the does the concentration of FtsZ until it reaches its
critical concentration. Since FtsZ is synthesized at a different rate from MinCD
or FtsA, the correct ratio of FtsZ with these factors is eventually reached.
This is supported by experimental evidence showing that cell division does not
proceed unless FtsZ is in a certain proportion with MinCDE and FtsA. Once the
required ratios are achieved, FtsZ polymerizes and, in coordination with MinE,
causes formation of the division ring at the mid-cell septum.
This model is
intriguing since it suggests a way in which mass could regulate division.
However, it reveals nothing about how the division factors evolved; in fact, the
model supports the contention that cytokinesis is a complex procedure involving
many interdependent factors. In addition, the model does not yet account for how
MinE actually promotes selection of the mid-cell septum. What it does imply,
however, is that FtsZ could have diverse activities. This is supported by the
fact that FtsZ works with several factors in addition to the ones we have
mentioned. For instance, there are a number of factors in addition to FtsA and
ZipA that operate at the mid-cell septum during cytokinesis and we will consider
these next.
Several studies have
shown that septum formation involves the coordinated interaction of several
proteins for the production of new membrane and cell wall components (Bramhill,
1997). FtsZ is thought to interact with several of these proteins at the septum
and is hypothesized to activate those involved in membrane and cell wall
synthesis. Figure 7 shows the proteins known to be involved at the septum and
their possible arrangement in the membrane. The number of components required
and the functions that must be performed at the septum is impressive. The
functions of the proteins and other factors required for cytokinesis are listed
in Table 2. Many are critical to cell division and cellular life. The
requirement of these factors and their regulation provides more evidence in
support of the hypothesis that in E.coli and perhaps other bacteria,
cytokinesis is an irreducibly complex system.
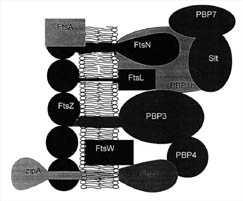
Figure 7. Proteins
present at the mid-cell septum in some bacteria. FtsZ works in conjunction with
several proteins at this site on the cytoplasmic membrane. FtsA and ZipA have
been shown to be required for FtsZ function in E.coli. The Penicillin binding
proteins (PBP) work together to synthesize cell wall components. The functions
of several other proteins are described in Table 2. (Based on Figure 8, Bramhill,
1997.)
But FtsZ-dependent cell
septum formation is not the only complex process involved in cytokinesis.
Another remarkable process that occurs is the precise partitioning of DNA
chromosomes to the new daughter bacteria. This is also known to be an active
process dependent on several critical protein factors.
Table 2. Several of
the proteins involved in cytokinesis
Partitioning of DNA during
cytokinesis
As we have noted, both
monoploidy and polyploidy are likely karyotypic outcomes of early protocell
evolution. Since monoploidy is the dominant form of genome structure found in
contemporary bacteria, the protocell theory must eventually account for the
dominance of the monoploid state in bacteria. Furthermore, in contemporary
bacteria the monoploid state is not achieved by random partition processes but
is postulated to involve an elaborate protein-dependent mechanism that results
in accurate partitioning of DNA chromosomes greater than 99.9 percent of the
time under optimal conditions. Recent evidence provides a remarkable picture of
how this protein-driven partitioning mechanism is involved in specifically
segregating bacterial DNA strands or chromosomes.
Three major problems
must be solved by the partitioning mechanism if the DNA chromosomes are to be
accurately distributed. (1) The circular DNA strands must be decatenated after
DNA replication. Decatenation involves the unlinking of two circular DNA
chromosomes that are catenated, which means they are linked together like two
links of chain. (2) Once DNA is decatenated, there must be a mechanism to
separate the strands and direct them to opposite poles of the mother cell. (3)
The decatenation and separation of the DNA must occur before the septum wall
forms and the daughter bacteria separate. Recent studies show that all three of
these problems are overcome by an active protein-dependent partition process
involving several proteins.
The partitioning
process has been observed to begin soon after DNA replication initiation in both
B.subtilis and E.coli (Levin and Grossman, 1998). The data
supporting a partitioning process that begins very early in the cell cycle comes
from the study of newborn daughter bacteria that have inherited a partially
replicated DNA chromosome bearing two DNA replication origins. Using fluorescent
tags attached to the origin regions, researchers have been able to observe the
two replication origins being actively pulled apart, each toward a cell pole. As
the cell cycle proceeds, the DNA finishes replication, is decatenated, and each
new DNA strand moves towards its origin (see Figure 8). Each new daughter DNA
then begins replication again in the mother cell before the completion of
cytokinesis. The new replication origins are eventually polarized on each newly
copied DNA strand such that one origin is directed toward the pole and one
toward the forming septum of the mother cell (see Figure 8). When the cell
divides, the two new daughter bacteria look similar to the parent cell, with the
replication origins of the DNA oriented toward the poles of the newborn cell. In
B.subtilis this partitioning phenomena is dependent on a protein produced
by a gene called SpoOJ (Levin and Grossman, 1998). Mutations in SpoOJ can cause
the formation of anucleate bacteria. SpoOJ protein is associated with the origin
site on the DNA. It is postulated that SpoOJ could operate like a tether that
actively segregates the DNA replication origins.
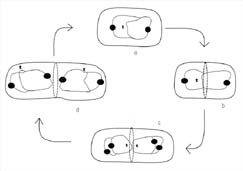
Figure 8. Active
partitioning of the bacterial chromosome before completion of cytokinesis. DNA
replication can occur very early in the cell cycle. The filled circles in the
diagram represent proteins that have been observed binding on the DNA near the
replication origin. (a) Researchers have noted that the origins are actively
polarized toward opposite ends of the cell by a protein-dependent mechanism. (b)
As the cell grows a septum begins to form and FtsZ begins to polymerize at
mid-cell. (c) DNA replication is completed but begins again on each new DNA. (d)
The replication origins are once again polarized by an active protein-dependent
process such that each new daughter cell receives a single, partially replicated
DNA chromosome with origins that are polarized towards the cell poles. (Drawing
is adapted from Levin and Grossman, 1998.) A similar figure is available for
viewing on the Internet in an animated form at http://www.cedarville.edu/dept/sm/jwf/division.htm.
This protein-dependent
segregation mechanism has also been confirmed to exist in both Caulobacter
crescentus and E.coli. A protein similar to SpoOJ has been identified
in C. crescentus but has not been yet identified in E.coli.
However, motor proteins that are involved in chromosome partitioning have been
identified in E.coli and B.subtilis (Hiraga, 1993). In E.coli a
protein produced by the mukB gene has been identified as a filamentous motor
protein that binds to DNA (Figure 9). It is hypothesized that mukB may act like
a cytoskeletal protein that actively moves the DNA, causing it to move toward
the pole towards which the origin binding protein has been directed. The role of
mukB in partitioning has been confirmed by observing bacteria with mutant mukB
genes. The mutant bacteria frequently divide abnormally, producing anucleate
bacteria and bacteria with two copies of DNA.
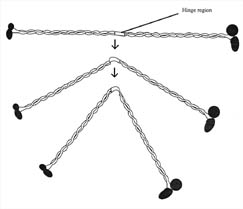
Figure 9. MukB
protein. Drawing of the putative structure of the mukB motor protein. Each
protein is made of several protein filaments and globular heads. The middle
hinge region may allow mukB to bend. MukB is involved in the partitioning and
packing of the bacterial chromosome during cell division. MukB has binding sites
for both FtsZ and DNA. (Redrawn from Figure 1, Hiraga, 1993.)
Curiously, FtsZ mutants
produce similar defects, suggesting that both FtZ and mukB protein may both be
involved in partitioning. In fact, recent studies have shown that mukB can bind
to FtsZ (Lockhart and Kendrick-Jones, 1998). These data suggest the exciting
possibility that these proteins may interact to coordinate chromosome
partitioning. Perhaps the relationship between mukB and FtsZ could account for
the apparent coordination between septation and partitioning, by insuring that
segregation occurs before septation. Thus, it appears that mukB and proteins
like it could be another essential part of an irreducibly complex
FtsZ-cytokinesis mechanism.
MukB is also known to
act with several other proteins to package the DNA (Hiraga, 1993). This sounds
like a simple feat but actually is a remarkable accomplishment, considering that
the DNA is approximately 1000 times longer than the bacterium itself. In fact,
each long circular DNA is packaged in a highly condensed state called the
nucleoid. This means the cell must untangle and decantenate two very long rings
of DNA, which involves untangling molecules that potentially have 200 or more
folds (Dillon, 1981). The decatenation process involves topoisomerases that
specifically perform the final separation process (see Figure 10). Additional
proteins then participate in folding the DNA into its highly condensed nucleoid
state so it can be packaged in the new daughter bacteria. Without the packaging
proteins and the topoisomerases, the nucleoids do not separate, which can
prevent cell division.

Figure 10. Decatentation
of the replicated bacterial chromosomes by topoisomerases. After DNA is
replicated, it is in a double-ringed, interlocked (catenated) form. There are
several topoisomerases in the bacterial cell that maintain chromosome structure.
In the case of decatenation, Topoisomerases IV binds to one of the DNA rings,
cuts it, and allows passage of the other ring through.
Decatenation of the DNA
rings may not be considered as complicated as some of the other processes, yet
it is one of the most critical events of cytokinesis. If the DNA does not
separate, production of two new daughter bacteria will not occur. Furthermore,
imagine the tremendous potential for error involved in decatenation of DNA in a
polyploid protocell. The problem of handling circular DNA was highlighted
recently in a speculative review paper on microbial evolution, in which the
authors challenge current dogma and claim that eukaryotes most likely evolved before
prokaryotes, because eukaryotes have a much more unsophisticated system for
replicating their genomes (primarily because the eukaryotic genome is linear and
not circular) (Pennisi, 1998; and Jeffares, 1998).
Adding to the
complexity of chromosome partitioning is the finding that bacterial DNA is
maintained in a specific condensed arrangement in the nucleoid. The condensed
state of the nucleoid is maintained by several protein factors, some of which
are essential to bacterial life (see Table 3). In fact, studies are revealing
that the nucleoid has a specific complex arrangement with the cell membrane. The
interaction between the membrane and nucleoid structure is thought to create a
channel-like substructure environment that harbors multi-enzyme complexes and
even controls insertion of proteins into the membrane. A group of investigators
are proposing that this complex substructure or “enzoskeleton” is a
necessary organelle-like structure that is essential to bacterial life (Norris
et al., 1996). Several other studies have shown that the interaction of factors
that form the nucleoid enzoskeleton are essential to maintain its shape and may
regulate transcription. Thus the nucleoid and its associated factors represent
another potentially irreducibly complex system.
Table 3. Some of the
proteins involved in maintenance of the nucleoid.
Conclusion
We have explored
several basic and essential processes involved in bacterial cell division. It
seems warranted to conclude that in some bacteria, both DNA replication and
cytokinesis are irreducibly complex. The presence of these complex systems in
bacteria—considered by many scientists to be “living fossils”—raises
questions about the gradual derivation of such systems in the pre-biotic soup or
the early protocell. Although the triggers and global regulators for these cell
division processes have not been elucidated, intriguing new evidence shows the
existence of factors coordinating activities such as DNA replication and
cytokinesis. For instance, a “response regulator” protein called CtrA has
been shown to regulate the cell cycle in C.crescentus by coordinating DNA
replication with cell division. CtrA , a transcription factor, modulates the
transcription of several cell-cycle promoters. Fascinatingly, CtrA is itself
subject to temporal and spatial control by both phosphorylation and proteolysis
(Shapiro, 1997). The presence of coordinating factors in bacteria like CtrA
supports the idea that bacterial cell division is irreducibly complex.
In summary:
- Bacterial cell
division appears to be irreducibly complex. There is evidence to suggest
that it involves multiple factors that are coordinated to interact precisely
with one another. For instance, it appears that the complex processes of DNA
replication, transcription, translation, cytokinesis, and chromosome
partitioning are interdependent and precisely coordinated during cell
division.
- The FtsZ-dependent
cytokinesis apparatus in E.coli fits the definition of an irreducibly
complex system because it involves several co-dependent parts that work
together like a machine. If any single part is eliminated, or its
concentration altered, cell division ceases or is aberrant. Therefore, we
can say that the gradual derivation of this system by natural selection
acting on a simple protocell is unlikely.
- Scientific evidence
gathered from the study of several free-living bacteria suggest the
existence of a common core cytokinesis system. The core system consists of a
division ring protein, a protein that directs the division ring to the
mid-cell septum, and a protein that helps bind the division ring to the
mid-cell septum. In addition, we can speculate that a protein that
partitions DNA strands may also be a part of this mechanism.
- Genome analysis has
revealed that some bacteria do not possess all the same proteins that are
present in the FtsZ-dependent cytokinesis apparatus of E.coli.
Therefore, a simpler, non-irreducibly complex apparatus may exist in these
bacteria. Alternately, a complex apparatus may exist, because all the
factors for cell division have yet to be discovered by functional analysis.
It is clear from these
conclusions that cell division is not a trivial or simple biological mechanism.
It will be exciting to see if an irreducibly complex cytokinesis apparatus is
universal among bacteria. The existence of a universal irreducibly complex
cytokinesis mechanism would challenge the validity of the protocell theory.
Because cell division is a complex mechanism in many bacteria, it is reasonable
to assume that even, in chlamydia and mycoplasma, a cytokinesis system exists
and that it is irreducibly complex. The existence of several different kinds of
irreducibly complex cytokinesis apparatuses (e.g., one that is FtsZ-independent)
would be even more problematic for protocell theorists to explain. Future
studies that focus on identifying cytokinesis factors by functional analysis
will be very helpful in determining the nature of the complexity of this
process. For instance, analysis of chlamydia for the presence of an endogenous
FtsZ-like protein, or one recruited from the host cell, will help determine if a
division ring protein is essential for division.
Acknowledgments:
This project was supported by awards from the Faculty Summer Scholarship Program
and the Computer Services Faculty Incentive Fund at Cedarville College.
NOTES:
1. The focus of this
paper is on the ability of biopolymers (proteins and nucleic acids) and
bio-synthetic pathways to work together in a coordinated fashion to create the
intricate clockwork-like conditions that promote cell division. I do not address
the question of whether these biopolymers themselves could have originated by a
gradualistic, evolutionary process in a prebiotic soup. This problem has been
addressed in several reviews (Swee-Eng, 1996, and Mills, 1996).
REFERENCES
Baumann P., and Jackson
S. “An archaebacterial homologue of the essential eubacterial cell division
protein FtsZ.” Proceeding of the National Academy of Science 93
(1996):6726-6730.
Behe, Michael. Darwin’s
Black Box. New York: The Free Press, 1996.
Bouche, J.P., and
Pichoff, S. “On the birth and fate of bacterial division sites.” Molecular
Microbiology 29 (1998):19-26.
Bramhill, D.
“Bacterial cell division.” Annual Review of Cell and Developmental
Biology 13 (1997):395-424.
Cooper, Geoffrey. The
Cell: A molecular Approach. Washington DC:ASM Press, 1997.
Dillon, S.
Ultrastructure, Macromolecules and Evolution. New York: Plenum Press,
1981.
Erickson H., et al.
“Bacterial cell division protein FtsZ assembles into protofilament sheets and
minirings, structural homologs of tubulin polymers.” Proceeding of the
National Academy of Science 93 (1996):519-523.
Hiraga, S.
“Chromosome partition in Escherichia coli.” Current Biology 5
(1993):789-801.
Javor, George. “Life
an evidence for creation.” Proceedings of the Fourth International
Conference on Creationism. Pittsburgh: Creation Science Fellowship, 1998.
Jeffares, D. “Relics
from the RNA world.” Journal of Molecular Evolution 46 (1998):18-36.
Koch, Arthur.
“Evolution vs. the number of gene copies per primitive cell.” Journal of
Molecular Evolution 20 (1984):71-76.
Levin, P., and
Grossman, A. “Cell cycle: the bacterial approach to coordination.” Current
Biology 8 (1998):R28-R31.
Lewin, Benjamin. Genes
VI. New York: Oxford University Press. 1997.
Lockhart A, and
Kendrick-Jones J. “Interaction of the N-terminal domain of mukB with the
bacterial tubulin homologue FtsZ.” FEBS Letters 430 (1998):278-282.
Loomis, William. Four
Billion Years. Sunderland Mass: Sinauer Associates, 1988.
Lutkenhaus, J. “Cell
division inhibitors SulA and MinCD prevent formation of the FtsZ ring.” Journal
of Bacteriology, 175 (1993):1118-1125.
Lutkenhaus, J.
“Escherichia coli cell division.” Current opinion in genetics and
development 3 (1993):783-788.
Ma, X. et al.
“Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal
structures in living Escherichia coli bacteria by using green fluorescent
protein.” Proceeding of the National Academy of Science 93
(1996):12998-13003.
Mills, G. and Kenyon D.
“The RNA world: A critique.” Origins and Design. 17 (1996):9-14.
Morowitz, Harold.
Beginning of Cellular Life. New Haven and London: Yale University Press,
1992.
Norris, V., Turnock,
G., and Sigee, D. “The Eschericia coli enzoskeleton.” Molecular
Microbiology 19 (1996):197-204.
Okada Y., et al.
“Possible function of the cytoplasmic axial filaments in chromosome
segregation and cellular division of Eschericia coli.” Science Progress
77 (1994):253-264.
Pennisi, E. “Direct
descendants from an RNA world.” Science 280 (1998):673.
Rhee, D. et al. “The
premyofibril: evidence for its role in myofibrillogenesis.” Cell Motility
and Cytoskeleton 28 (1994):1-24.
Sanger, Joseph.
Personal web page. Retrieved Feb 15, 1999 from the world wide web (http://www.med.upenn.edu/~ifem/js.htm).
Schramm, N., and Wyrick,
P.B. “Cytoskeletal requirements in Chlamydia trachomatis infection of host
cells.” Infection and Immunity 63 (1995):324-332.
Shapiro, L., and Losick,
R. “Protein localization and cell fate in bacteria.” Science 276
(1997):712-718.
Stephens, R.S.
“Genome sequence of an obligate intracellular pathogen of humans: Chlamydia
trachomatis.” Science 282 (1998):754-759.
Swee-Eng, A. “The
Origin of Life: A critique of current scientific models.” Creation Ex
Nihilo Technical Journal 10 (1996):300-314.
Vincente, M., and
Errington, J. “Structure, function and controls in microbial division.” Molecular
Microbiology 20 (1996):1-7.
Vinella, D. and D’Ari,
R. “Overview of controls in the Escherichia coli cell cycle.” BioEssays 17
(1995):527-536.
Copyright 2000 Access
Research Network. All rights reserved. International copyright secured.
File Date: 6.27.01

 From the Access Research Network
From the Access Research Network




![]()
![]()
![]()